Making Tumors Visible & Accesible So Immunotherapy Can Work
A single-asset, tumor-targeted dual-payload antibody–oligonucleotide conjugate (AOC) designed to simultaneously modulate the two core intracellular drivers of immunotherapy resistance.
Founder-led and capital-efficient, we are advancing one focused program with a clear preclinical path to the clinic and defined value inflection points.
Strategic Partners & Recognition






Expanding Treatment Options for Immunotherapy-Resistant Colorectal Cancer
Approximately 85% of colorectal cancer patients fail to respond to immunotherapy due to two fundamental barriers: tumor invisibility caused by poor antigen presentation, and immune exclusion that prevents immune cell access. Current investigational therapies, even in the best-case scenario, address only one of these barriers—or neither—limiting durable responses. Colorectal cancer is therefore a highly strategic starting point, with well-defined immune resistance biology and clear clinical endpoints that enable efficient demonstration of mechanism and increase the likelihood of clinical success.
Solution
Scientific Differentiation
Technology & Expansion Beyond RNA & Oncology
Built for Focus Execution & Value Creation
Sebastian BioPharma is a capital-efficient, founder-led biotech advancing a single lead asset with a clear path from preclinical validation to clinical development. Founded by leaders in RNA therapeutics and tumor immunology, the company is built on more than a decade of peer-reviewed research, with exclusive IP from the University of Miami and an industry-aligned, GMP-compatible development strategy.
SBP-001 is currently in hit-to-lead development with validated in-vitro and in-vivo proof of concept, supported by $630K of founder capital. We are closing an $870K SAFE to complete a $1.5M pre-seed round, advancing toward lead candidate nomination and a seed-ready, IND-enabling package focused on value creation at defined inflection points.
We welcome engagement with scientific angels, early-stage funds, and individuals personally motivated to expand treatment options for patients with cancer.
Silencing TAP Makes Tumor Louder
Featured by the American Committee for Immunology Research (ACIR), this article highlights Sebastian Biopharma’s approach to reprogramming the tumor microenvironment throught TAP silencing. The result: enhanced tumor visibility, potent antitumor effects in both mouse and human preclinical models, and promising synergy with immune checkpoint blockade—without added toxicity.
Innovation Grounded in Peer-Reviewed Science
Sebastian BioPharma’s approach is grounded in more than a decade of peer-reviewed research in tumor immunology and RNA-based therapeutics. This scientific foundation underpins the design of SBP-001 and supports its translational relevance in immune-resistant solid tumors.

Nature Communications, 2019
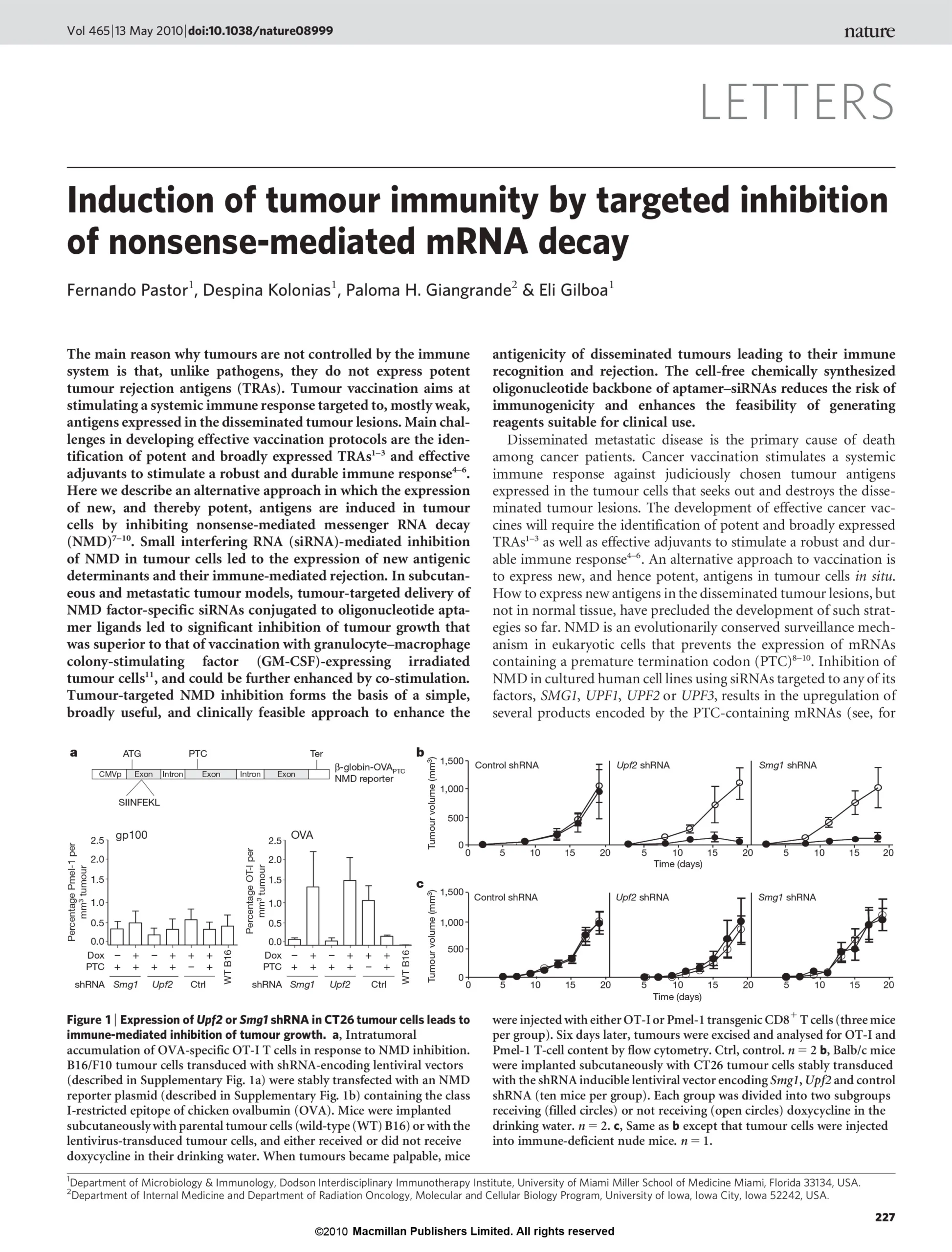
Nature , 2010

Cancer Inmunology Research, 2020
Research Features, 2021

Vaccination against neoantigens induced in cross-priming cDC1 in vivo

The Journal of Infectious Diseases, 2025
Driven by Purpose Led by Experts
Sebastian BioPharma is led by a founding team with deep expertise in RNA therapeutics, immuno-oncology, and company building, combining scientific leadership with hands-on execution. The team is complemented by advisors supporting business development, partnering, and commercialization.

Eli Gilboa, PhD
CSO & Founder
Scientific leader in tumor neoantigen & RNA therapeutics. Multiple grant awarded for Sebastian Bio’s pipeline.

Eli Gilboa, PhD
CSO & Founder
- Teléfono:+1 (859) 254-6589
- Correo electrónico:info@example.com

Greta Garrido, PhD
CEO & Co-Founder
Biotech executive driving IO drug development & Strategic growth. Led 2 FDA approvals >50M in successful fundraising.

Greta Garrido, PhD
CEO & Co-Founder
- Teléfono:+1 (859) 254-6589
- Correo electrónico:info@example.com

Brett Schrand, PhD
Research & Development, Associated Director
Oligonucleotide therapeutics discovery and development. Hands-on CRO execution across in-vitro and in-vivo studies.

Brett Schrand, PhD
Research & Development, Associated Director
- Teléfono:+1 (859) 254-6589
- Correo electrónico:info@example.com
Turning Proof into Progress
Our progress is backed by execution, not aspiration.
Exclusive University of Miami IP plus company-owned filings securing freedom to operate.
Advanced SBP-001 through validated in-vitro/in-vivo proof of concept into hit-to-lead.
High-impact oncology and immunology journals supporting the underlying biology.
Investment
Strategic Investment Opporunity: Expanding Immunotherapy Acces
SBP-001 is designed to expand immunotherapy access for patients with limited treatment options, using a dual-payload AOC to address tumor-intrinsic immune resistance in a single, scalable drug.
- Closing an $870K SAFE to complete a $1.5M pre-seed round.
- Funds lead SBP-001 candidate nomination and preparation of a seed-ready, IND-enabling package.
Want to Join our Mission?Let´s Talk
We’re building strategic partnerships with investors, collaborators and institutions ready to accelerate the future of oncology.
Drop us an email
contact@sebastianbio.com
Address
100 Cumming Center Suite 451-C Beverly MA 01915
